Description
WEGOVY® (semaglutide) injection 0.5 mg is an injectable prescription medicine that may help adults and children aged ≥12 years with obesity (BMI ≥30 for adults, BMI ≥ 95th percentile for age and sex for children), or some adults with excess weight (BMI ≥27) (overweight) who also have weight-related medical problems to help them lose weight and keep it off. Wegovy® should be used with a reduced calorie meal plan and increased physical activity
Wegovy® contains semaglutide and should not be used with other semaglutide-containing products or other GLP-1 receptor agonist medicines
It is not known if Wegovy® is safe and effective when taken with other prescription, over-the-counter, or herbal weight loss products
It is not known if Wegovy® can be used safely in people with a history of pancreatitis
It is not known if Wegovy® is safe and effective for use in children under 12 years of age.
Wegovy® is the first and only prescription weight-management medicine taken once weekly. It’s taken on the same day each week, any time of day, with or without food.
Wegovy® is available in 5 doses ranging from 0.25 mg to 2.4 mg, each of which is taken once a week. Each dose comes in a different color pen.
 Cart is empty
Cart is empty 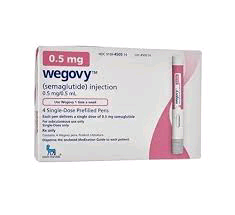




Samuelson Jessica (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Henrietta Adams (verified owner) –
Good service all round from customer service to delivery service and refund services thank you.
Almond Bryan (verified owner) –
Very well worth the money thank you.
Daniela (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
Kayden François (verified owner) –
Very well worth the money thank you.
Andy Nicholas (verified owner) –
Very well worth the money thank you.
Max Senna (verified owner) –
Very well worth the money thank you.
Roberta Miller (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Jessica Boland (verified owner) –
Good quality in all their products especially botulinums toxin products.
Anderson Tyler (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Mason Moora (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
Stella Williams (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Helene Matthews (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Helene Matthews (verified owner) –
Very well worth the money thank you.
Diana Zohar (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
George Owen (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
Kévin Gameiro (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
Stella Williams (verified owner) –
Good quality in all their products especially botulinums toxin products.
Diana Zohar (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Lordan Jenna (verified owner) –
Very fast delivery less than 4 days specially for me staying almost at the other end of the world.
Stella Patrick (verified owner) –
Very well worth the money thank you.
Avery Maiden (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Henrietta Adams (verified owner) –
Very well worth the money thank you.
Stella Williams (verified owner) –
The product is firmly packed and we’ll handled and always delivered in great condition.
Hayden Melissa (verified owner) –
Very well worth the money thank you.
Martha Stewart (verified owner) –
Very fast delivery less than 4 days specially for me staying almost at the other end of the world.
Kayden François (verified owner) –
Good quality in all their products especially botulinums toxin products.
Kévin Gameiro (verified owner) –
Good service all round from customer service to delivery service and refund services thank you.
Leo Lagrange (verified owner) –
I’m glad my colleague referred you to me, because I was finding it difficult getting the best quality products available and at a moderate prices too.
Bryan McPherson (verified owner) –
Very fast delivery less than 4 days specially for me staying almost at the other end of the world.